New York
The Trump administration has approved a USD 1.6 million contract without competitive bidding for a Danish university to conduct research on hepatitis B vaccination among newborns in Africa, a decision that has triggered serious ethical concerns among public health experts.
The contract was granted to a research group whose past work has frequently been cited by anti-vaccine campaigners and criticised by several leading scientists. Health experts have warned that the proposed study could be unethical, as it involves withholding a proven and effective vaccine from infants who face a high risk of infection.
The Associated Press has learned that the study did not undergo the standard ethics review process typically required for such federally funded research.
The Centres for Disease Control and Prevention awarded the funding to a team at the University of Southern Denmark, a group that has publicly received praise from US Health Secretary Robert F. Kennedy Jr., according to a federal notice released this week.
One of the principal investigators, Christine Stabell Benn, also serves as a consultant to a Kennedy-appointed advisory panel that recently voted to discontinue the recommendation for a universal hepatitis B vaccine dose for newborns in the United States.
The research is scheduled to begin early next year in Guinea-Bissau, a low-income West African country where hepatitis B infections remain widespread. The five-year study plans to enrol approximately 14,000 newborns.
Designed as a randomised controlled trial, the project will assign some infants to receive the hepatitis B vaccine at birth while others will not. Researchers will monitor participants for mortality, illness and long-term developmental outcomes.
While most children will be followed for less than two years to track potential side effects, the first 500 enrolled infants will be observed for up to five years to assess neurological and behavioural development. According to a study protocol prepared earlier this year and obtained by the AP, the trial does not include a placebo group.
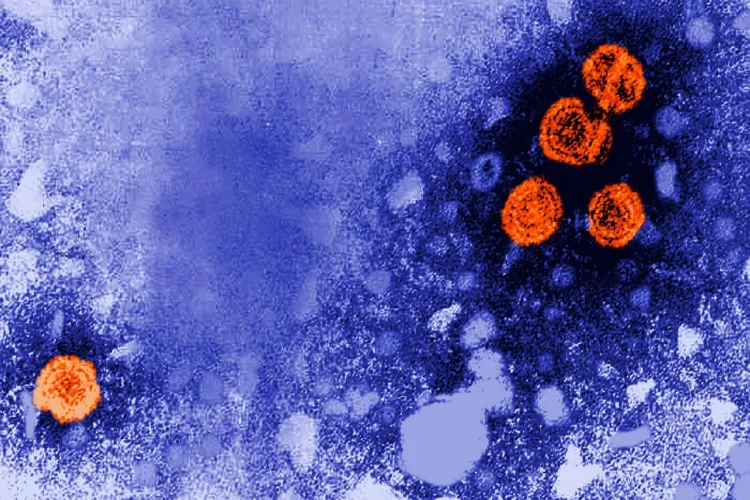
Hepatitis B can be transmitted from mother to child at birth and also through close contact with infected individuals. Decades of medical evidence show that early vaccination effectively protects infants from chronic liver disease and premature death.
Because the vaccine’s benefits are well-established, experts say deliberately withholding it from some babies — particularly Black infants in a high-risk setting — raises profound ethical red flags.
Dr Boghuma K Titanji, an infectious disease specialist at Emory University, said the risks of infection vastly outweigh any unproven concerns about side effects.
She described the study as “unconscionable” and warned that it could deepen vaccine hesitancy across Africa and beyond.
“There is enormous potential for harm here,” said Titanji, who is originally from Cameroon.
Benn did not respond to an email seeking comment. An automated reply indicated she is out of the office until early January. However, the research team said in a statement that the project “will be the first and likely the only study of its kind.”
The researchers argue that the trial takes advantage of a limited policy window. Guinea-Bissau does not currently mandate a hepatitis B birth dose but plans to introduce universal newborn vaccination in 2027.
Vaccine sceptics have long claimed that possible side effects of the hepatitis B vaccine were insufficiently studied before the CDC recommended it for newborns in 1991. Public health authorities counter that more than 30 years of data have shown no serious long-term adverse effects.
The circumstances surrounding the contract award are highly unusual. The CDC did not issue a call for research proposals, and the project bypassed its customary peer and ethics review process.
According to a CDC official familiar with the matter, the proposal was unsolicited, and agency leadership was instructed by the Department of Health and Human Services to approve it, with assurances that special funding would be provided.
The official, speaking anonymously because they were not authorised to comment publicly, said internal communications among CDC staff reflected anger and alarm over the decision.
Some agency scientists privately compared the study to the infamous Tuskegee syphilis experiment, in which Black men were denied treatment so doctors could observe disease progression.
Like Tuskegee, the current proposal would involve researchers observing preventable illness in vulnerable populations, Titanji said.
“The comparison is appropriate,” she added.
The research team has said the trial was approved by Guinea-Bissau’s national ethics committee. However, the CDC did not conduct its own formal ethics review, the agency official confirmed.
In a statement, HHS spokesperson Andrew Nixon said the department would ensure that “the highest scientific and ethical standards are met.”
Public health experts noted that Benn and her husband, Peter Aaby, have previously faced scrutiny for research conducted through their Bandim Health Project.
Other Danish scientists who have reviewed their work have raised concerns about research methods. Earlier this year, former CDC director Dr Tom Frieden wrote an editorial describing a 2017 study co-authored by Benn and Aaby as “fundamentally flawed.”
Several researchers sharply criticised the latest funding decision.
Carl Bergstrom, an evolutionary biologist at the University of Washington, wrote on social media that the study amounted to “a hepatitis B vaccine deprivation trial” and questioned whether political influence had played a role.
Dr Angela Rasmussen, a virologist at the University of Saskatchewan, said the decision amounted to handing taxpayer money to political allies for a “grossly unethical study” that would expose African infants to unnecessary risk.
READ MORE: Ashhar-Anil dosti: A bond that transcends religions
Jeffrey Epstein, a wealthy financier and convicted sex offender, was found dead in his jail cell in 2019 while awaiting trial on federal sex-trafficking charges. His death was ruled a suicide, though it continues to generate controversy and speculation.